Date: 21 January 2021
The delivery of wound care in the current climate is challenging so effective solutions are more important than ever. It is vital to be aware of the latest products and innovations that have the potential to improve outcomes.
Topics: Chronic wounds
The clinical challenges of chronic wounds
Highly exuding chronic wounds, including cavities, can present a number of clinical management challenges. Infection and biofilm are common complications of chronic wounds that can delay healing and result in increased exudate production which, if managed inappropriately, can lead to maceration of the peri-wound area, resulting in wound enlargement (Bajjada, 2017; WUWHS, 2019). The presence of devitalised tissue within the wound can also lead to an increased risk of infection, biofilm development, delayed healing and increased exudate volume (WUWHS, 2019).Exufiber® and Exufiber® Ag+ are a new generation of gelling fibre dressings that can be used to address many of these challenges in chronic wounds, to optimise conditions for healing.
What is Exufiber® and Exufiber® Ag+?
Exufiber® and Exufiber® Ag+ dressings contain next generation gelling fibres and use Hydrolock® Technology to optimise wound healing conditions.Hydrolock® Technology
Hydrolock® Technology uses tightly packed, non-woven polyvinyl alcohol fibres which transform to a gel on contact with wound exudate.This gives Exufiber® the ability to absorb and retain a large volume of exudate, avoiding leakage and associated skin damage. The dressing also retains fluid when used under compression therapy (Smet et al, 2015; Chadwick and McCardle, 2016; Davies and McCarty, 2017). If a secondary dressing is needed, the Exufiber® dressing range uses capillary action to transfer exudate away from the wound bed into the secondary dressing (Chadwick and McCardle, 2016; Molnlycke Health Care, 2018).
Exufiber® has a high tensile strength that enables it to stay intact, facilitating one piece removal (Figure 1), which is of particular benefit in cavity wounds, ensuring no dressing fragments are left behind. Despite its tensile strength, Exufiber® is soft and conformable, making it easy to apply and remove, and maximising contact with the wound bed to avoid leakage (Smet et al, 2015; Chadwick and McCardle, 2016; Davies and McCarty, 2017).
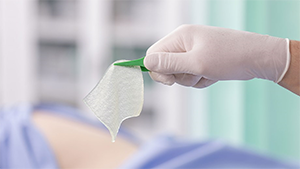 Figure 1. One piece removal.
Figure 1. One piece removal.Mode of action: how does Exufiber® and Exufiber® Ag+ work?
Exufiber® and Exufiber® Ag+ addresses the clinical challenges of chronic wounds in three ways.
1. Efficient transfer of exudate
The ability of a primary dressing to absorb and transfer exudate to a secondary dressing is important to prevent pooling, leakage and maceration (Mölnlycke Health Care, 2014; Chadwick and McCardle, 2016). Exufiber® is able to transfer exudate efficiently from the wound bed to a secondary dressing (Chadwick and McCardle, 2016; Mölnlycke Health Care, 2018;2020a).The dressing can be left in place for a maximum of 7 days, and up to 14 days on donor sites, depending on wound conditions, to leave the wound bed undisturbed (Mölnlycke Health Care, 2014; Hamberg et al, 2017).

2. Supports a clean wound bed
Exufiber® dressings breakdown slough and remove debris and biofilm by promoting autolytic debridement (Figures 2 and 3), absorbing and retaining exudate and micro-organisms (Smet et al, 2015). These actions help to promote a clean wound bed (Figure 4) which is essential for wound healing to occur (Atkin et al, 2019).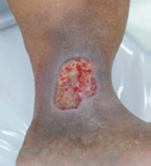
Figure 2. Before debridement.

Figure 2. Before debridement.
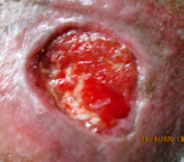
Figure 4. A clean wound bed.
3. Prevents biofilm reformation
Exufiber® Ag+ combines the benefits of Hydrolock® Technology with antimicrobial action. The dressing contains fine silver sulphate crystals which are embedded throughout the dressing and which dissolve on contact with exudate, releasing silver ions which exert an antimicrobial effect on micro-organisms present in the wound bed.The antimicrobial action of Exufiber® Ag+ is rapid and sustained, starting three hours following application and lasting for up to seven days, giving protection against a broad range of pathogens (Mölnlycke Health Care, 2016; Hamberg et al, 2017).
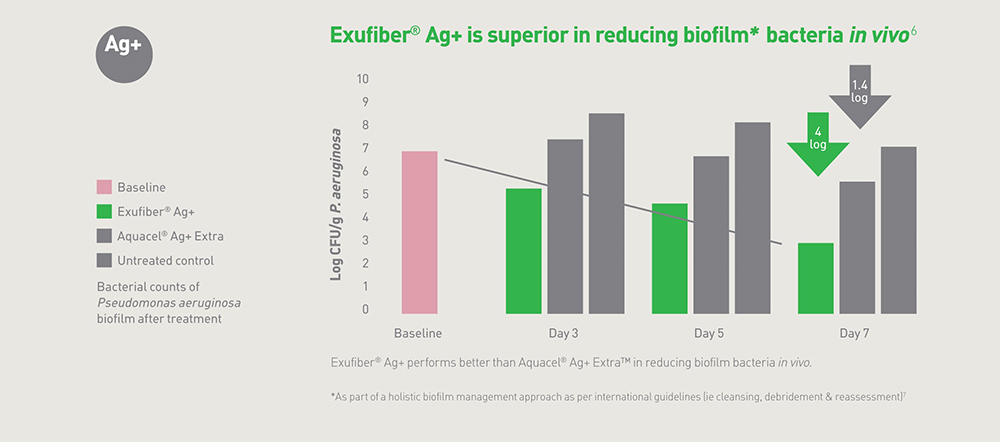 Figure 5. The in vivo reduction of biofilm bacteria using Exufiber ® Ag+ as part of a biofilm management approach.
Figure 5. The in vivo reduction of biofilm bacteria using Exufiber ® Ag+ as part of a biofilm management approach.These features are summarised in a short video: www.molnlycke.co.uk/products-solutions/exufiber-ag-plus
Biofilms are known to be present in almost all chronic, non-healing wounds (Atkin et al, 2019). Exufiber® Ag+ has also been shown to reduce biofilm bacteria (Figure 5) and prevent reformation in vivo and can be used as part of a biofilm management approach (Gil et al, 2017; Davis et al, 2019).
Patient experience
The correct management of heavily exuding wounds, including cavities, is important to minimise the impact of the wound on the patient’s quality of life. Heavily exuding woundsBiofilms are known to be present in almost all chronic, non-healing wounds (Atkin et al, 2019). Exufiber® Ag+ has also been shown to reduce biofilm bacteria (Figure 5) and prevent reformation in vivo and can be used as part of a biofilm management approach (Gil et al, 2017; Davis et al, 2019).
The correct management of heavily exuding wounds, including cavities, is important to minimise the impact of the wound on the patient’s quality of life. Heavily exuding wounds
Patient experience
The correct management of heavily exuding wounds, including cavities, is important to minimise the impact of the wound on the patient’s quality of life. Heavily exuding wounds
Clinical evidence for Exufiber® and Exufiber® Ag+
A recent randomised, controlled trial (Mölnlycke Health Care, 2020b) of 248 venous leg ulcer patients found that Exufiber® outperformed Aquacel® Extra™ across multiple measures. These included a better wound size reduction, clinician satisfaction for overall experience of use, ease of removal, and non-adherence to wound bed, and clinicians reported better absorption and lock in of exudate, blood and slough.In an in-use product evaluation (Davies and McCarty, 2017), a total of 320 questionnaires were returned for analysis. A variety of wound types had been treated with Exufiber® including leg ulcers, pressure ulcers and diabetic foot ulcers. Exufiber® was rated highly in terms of exudate management, including the dressing’s absorption and retention capacity. Most respondents rated Exufiber® as either ‘very good’ or ‘good’ in terms of conformability and ease of removing the dressing in one piece. Overall, the clinicians were highly impressed with the performance of Exufiber® (Davies and McCarty, 2017).
In a 12-week study that evaluated the performance and safety of Exufiber® in highly exuding diabetic foot ulcers (Chadwick and McCardle, 2016), the authors reported an increased number of patients with healthy periwound skin and a steady decrease in exudate and wound size over the study period. There was a gradual increase in the amount of epithelial tissue indicating healing and a consistent low volume of sloughy tissue indicating an ability to promote a clean wound bed through the process of autolysis.
Two studies (Gil et al, 2017; Davis et al, 2019) demonstrated the ability of Exufiber® Ag+ to prevent biofilm reformation by reducing the number of micro-organisms in in vivo tests. The tests also compared results with Aquacel® AG+ Extra™ and used both MRSA and Pseudomonas Aeruginosa biofilm (Figure 5).
The superior lock in capacity of exudate was demonstrated in testing performed by Surgical Materials Testing Laboratory (SMTL) (Mölnlycke Health Care, 2014) when compared with Aquacel®, Aquacel® Extra™, Durafiber® and UrgoClean®.
Bajjada T (2017) Using a step-up, step-down approach to exudate management. J Comm Nurs 31(2): 32–8
Chadwick P, McCardle J (2016) Open, non-comparative, multicentre post clinical study of the performance and safety of a gelling fibre wound dressing on diabetic foot ulcers. J Wound Care 25(5): 290–300
Davies P, McCarty S (2017) An in-use product evaluation of a gelling fibre dressing in wound management. E-poster presentation at Wounds UK Conference, Harrogate, UK
Davis SC, Li J, Gil J, et al (2019) Preclinical evaluation of a novel silver gelling fiber dressing on Pseudomonas aeruginosa in a porcine wound infection model. Wound Rep Reg 27(4): 360–65
Gil J, et al (2017) Evaluation of a Gelling Fiber Dressing with Silver to Eliminate MRSA Biofilm Infections and Enhance the Healing. Poster presented at the Symposium on Advanced Wound Care Spring meeting/Wound Healing Society Annual Meeting, San Diego, CA, USA
Hamberg K, Gerner E, Falkbring S (2017) Antimicrobial effect of a new silver-containing gelling fibre dressing against common wound pathogens. Poster presented at the Symposium on Advanced Wound Care Spring meeting/Wound Healing Society (WHS) Annual Meeting 2017
McGrath A (2011) Overcoming the challenge of overgranulation. Wounds UK 7(1): 42–9
Mölnlycke Health Care (2014) SMTL TM-404 free swell absorption and retention. Data on file
Mölnlycke Health Care (2016) Performance of Exufiber® Ag+ in vitro; Antimicrobial effect, silver release kinetics and minimal effective concentration. Data on file.
Mölnlycke Health Care (2018) Data on file.
Mölnlycke Health Care (2019) Exufiber® Ag+: Physical properties over time. Data on file.
Mölnlycke Health Care (2020a) Data on file.
Mölnlycke Health Care (2020b). Data on file.
Smet S, Beele H, Saine L, et al (2015) Open, non-comparative, multi-centre post market clinical follow-up investigation to evaluate performance and safety on pressure ulcers when using a gelling fibre dressing as intended. Poster Presentation at European Pressure Ulcer Advisory Panel Conference, Ghent, Belgium
World Union of Wound Healing Societies (2019) Consensus document. Wound exudate: effective assessment and management. Available online: www.woundsinternational.com
In a 12-week study that evaluated the performance and safety of Exufiber® in highly exuding diabetic foot ulcers (Chadwick and McCardle, 2016), the authors reported an increased number of patients with healthy periwound skin and a steady decrease in exudate and wound size over the study period. There was a gradual increase in the amount of epithelial tissue indicating healing and a consistent low volume of sloughy tissue indicating an ability to promote a clean wound bed through the process of autolysis.
Two studies (Gil et al, 2017; Davis et al, 2019) demonstrated the ability of Exufiber® Ag+ to prevent biofilm reformation by reducing the number of micro-organisms in in vivo tests. The tests also compared results with Aquacel® AG+ Extra™ and used both MRSA and Pseudomonas Aeruginosa biofilm (Figure 5).
The superior lock in capacity of exudate was demonstrated in testing performed by Surgical Materials Testing Laboratory (SMTL) (Mölnlycke Health Care, 2014) when compared with Aquacel®, Aquacel® Extra™, Durafiber® and UrgoClean®.
References
Atkin L, Bućko Z, Conde Montero E, et al (2019) Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care 28(Suppl3): S1–S51Bajjada T (2017) Using a step-up, step-down approach to exudate management. J Comm Nurs 31(2): 32–8
Chadwick P, McCardle J (2016) Open, non-comparative, multicentre post clinical study of the performance and safety of a gelling fibre wound dressing on diabetic foot ulcers. J Wound Care 25(5): 290–300
Davies P, McCarty S (2017) An in-use product evaluation of a gelling fibre dressing in wound management. E-poster presentation at Wounds UK Conference, Harrogate, UK
Davis SC, Li J, Gil J, et al (2019) Preclinical evaluation of a novel silver gelling fiber dressing on Pseudomonas aeruginosa in a porcine wound infection model. Wound Rep Reg 27(4): 360–65
Gil J, et al (2017) Evaluation of a Gelling Fiber Dressing with Silver to Eliminate MRSA Biofilm Infections and Enhance the Healing. Poster presented at the Symposium on Advanced Wound Care Spring meeting/Wound Healing Society Annual Meeting, San Diego, CA, USA
Hamberg K, Gerner E, Falkbring S (2017) Antimicrobial effect of a new silver-containing gelling fibre dressing against common wound pathogens. Poster presented at the Symposium on Advanced Wound Care Spring meeting/Wound Healing Society (WHS) Annual Meeting 2017
McGrath A (2011) Overcoming the challenge of overgranulation. Wounds UK 7(1): 42–9
Mölnlycke Health Care (2014) SMTL TM-404 free swell absorption and retention. Data on file
Mölnlycke Health Care (2016) Performance of Exufiber® Ag+ in vitro; Antimicrobial effect, silver release kinetics and minimal effective concentration. Data on file.
Mölnlycke Health Care (2018) Data on file.
Mölnlycke Health Care (2019) Exufiber® Ag+: Physical properties over time. Data on file.
Mölnlycke Health Care (2020a) Data on file.
Mölnlycke Health Care (2020b). Data on file.
Smet S, Beele H, Saine L, et al (2015) Open, non-comparative, multi-centre post market clinical follow-up investigation to evaluate performance and safety on pressure ulcers when using a gelling fibre dressing as intended. Poster Presentation at European Pressure Ulcer Advisory Panel Conference, Ghent, Belgium
World Union of Wound Healing Societies (2019) Consensus document. Wound exudate: effective assessment and management. Available online: www.woundsinternational.com




