Date: 06 October 2020
The delivery of wound care in the current climate is challenging so effective solutions are more important than ever. It is vital that you are aware of the latest products and innovations that have the potential to improve outcomes.
Topics: Wound care
Wounds, particularly those that are chronic, can result in signs and symptoms such as exudate and pain, which in turn may give rise to psychological issues, such as stress, sleep disturbance, a negative mood and social isolation (Upton, 2014; Brown, 2015).
Dressing removal is recognised as a cause of wound-related pain (Upton and Solowiej, 2010). There are several factors that can contribute to painful dressing removal, including aggressive adhesives, dried-out dressing materials and dried-out wound exudate (WUWHS, 2007). A recent international consensus document described how the removal of adhesive medical devices such as wound dressings can cause significant pain and distress, as well as resulting in delayed wound healing, increased risk of infection and increased costs of management (Fumarola et al, 2020). As a consequence, Fumarola et al (2020) recommended that healthcare professionals should incorporate pain-minimising strategies, such as the use of silicone wound dressings and skin barriers into their routine practice.
Dressing removal is recognised as a cause of wound-related pain (Upton and Solowiej, 2010). There are several factors that can contribute to painful dressing removal, including aggressive adhesives, dried-out dressing materials and dried-out wound exudate (WUWHS, 2007). A recent international consensus document described how the removal of adhesive medical devices such as wound dressings can cause significant pain and distress, as well as resulting in delayed wound healing, increased risk of infection and increased costs of management (Fumarola et al, 2020). As a consequence, Fumarola et al (2020) recommended that healthcare professionals should incorporate pain-minimising strategies, such as the use of silicone wound dressings and skin barriers into their routine practice.
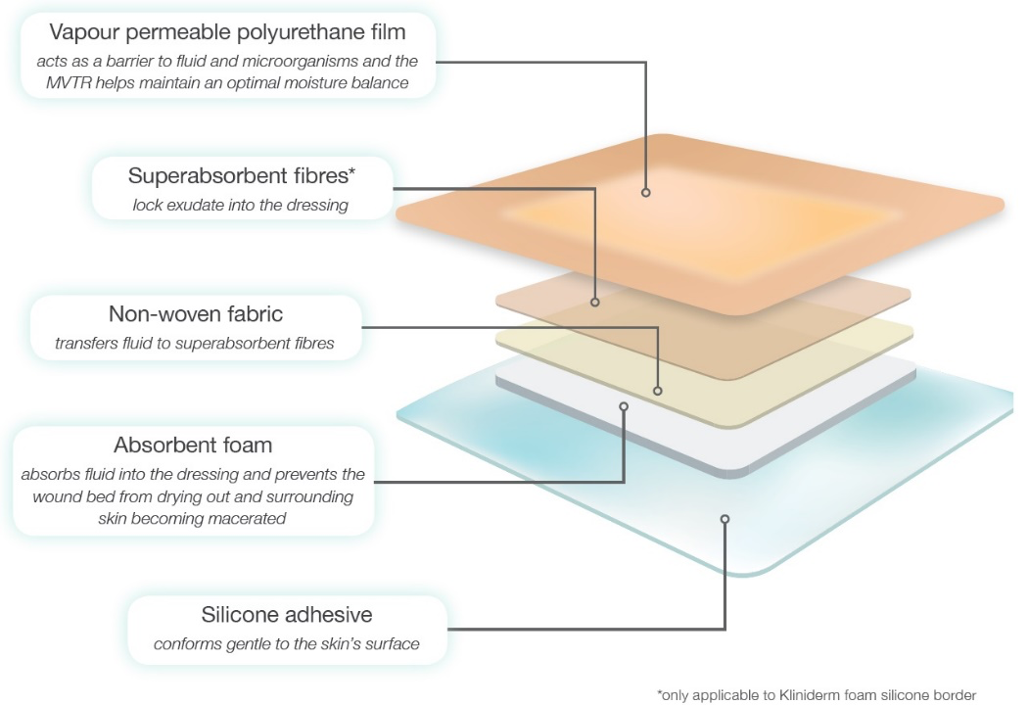
Figure 1. The five layers of Kliniderm® foam silicone.
What is Kliniderm® foam silicone?
Kliniderm® foam silicone is a soft, conformable absorbent polyurethane foam dressing with an adhesive silicone wound contact layer and a moisture permeable film backing. It is designed to minimise pain at dressing change, and is available in a normal or lite format, with or without a border.
Kliniderm® foam silicone comprises of five layers (Figure 1).
Kliniderm® foam silicone can be used in a wide variety of wound types. It is indicated for use in chronic wounds, e.g. pressure ulcers, diabetic foot ulcer and leg ulcers, and acute wounds, e.g. post-operative wounds, superficial and partial thickness burns, skin abrasions and skin tears.
Kliniderm® foam silicone lite is indicated for wounds with a low volume of exudate while Kliniderm® foam silicone can be used in wounds with a low to moderate exudate volume.
What are the key benefits?
The key benefits of using Kliniderm® foam silicone are:Gentle silicone adhesive: In vitro testing of the force needed to remove Kliniderm® foam silicone demonstrated that the amount of force required was equivalent to that of other market-leading silicone products (Carney et al, 2015).
Clinically, the silicone wound contact layer reduces the risk of pain during dressing removal, making the procedure more comfortable for the patient. It also helps to minimise the risk of medical adhesive-related skin injuries which is important to avoid associated complications such as infection and delayed healing (Fumarola et al, 2020). Kliniderm® foam silicone is easy to apply and remove and is conformable and comfortable (Drewery, 2015).
Absorbency: The foam, non-woven fabric and superabsorbent fibres (the latter in Kliniderm® foam silicone border only) enables the Kliniderm® foam silicone dressing range to manage a low to moderate volume of wound exudate (Carney et al, 2015). Kliniderm® foam silicone is also effective under compression therapy in the management of venous leg ulcers (Carney et al, 2015).
The absorbency of the range promotes moist wound healing, protects the periwound skin and can provide up to seven days of wear time. Kliniderm® foam silicone border is also showerproof which may further increase wear time and minimise the number of dressing changes needed.
Cost and clinical effectiveness: Kliniderm® foam silicone is a cost-effective alternative to other silicone dressings and has the potential to save up to 29% on silicone dressing spend when compared to other market leading brands (Drug Tariff, 2020*). This could result in significant cost savings for organisations (Drewery, 2015).
*Prices correct from September 2020, based on closest comparable size.
In a multi-centre evaluation of the Kliniderm® foam silicone dressing range, 97% of participating clinicians found that the dressings had equal or better performance than the current formulary product, using the parameters of patient comfort, conformability, exudate management and ease of application and removal. Furthermore, 95% of clinicians stated that they would recommend Kliniderm® for formulary inclusion, based on dressing performance (Data on File, 2020).
The Kliniderm® foam silicone dressing range includes a comprehensive selection of sizes and shapes, including sacral and heel (Figure 2).


Figure 2. The Kliniderm® foam silicone dressing range.
What is the evidence for using Kliniderm® foam silicone?
A multi-centre evaluation of Kliniderm® foam silicone dressings was carried out across five different clinical sites to determine the suitability of the range to address both patient and nursing needs (Data on file, 2020). The parameters of comfort, conformability, exudate management and ease of application and removal were evaluated on 60 patients with a variety of acute and chronic wounds. Results from 206 dressing changes showed that 97% of clinicians rated the performance of Kliniderm® foam silicone as equal to, or better than, the current formulary product, while 93% of patients rated dressing comfort as good or very good (Data on file, 2020). More information can be found here.Rafter et al (2016) state that silicone dressings are often used to provide comfort, facilitate an optimal wound environment and to minimise skin damage. To determine if there was clinical efficacy and cost-effectiveness, they compared four silicone dressings, including Kliniderm® foam silicone, in an 80 patient evaluation of skin tear wounds in a nursing home setting (Rafter et al, 2016). Following education for trained and untrained staff they received a first aid skin tear kit containing the necessary products and the silicone dressing to be evaluated as a primary dressing. There was no difference in wound healing rates between the four silicone dressings and Kliniderm® foam silicone stayed in place well. All of the silicone dressings used in the audit resulted in positive clinical outcomes with healing or progression to healing in all cases.
Drewery (2015) undertook a clinical evaluation of Kliniderm® foam silicone dressing compared to two market leading silicone foam dressing products on formulary. The evaluation took place in nine district nurse teams and involved 22 patients with wounds producing a light to moderate exudate volume for eight dressing changes during a six-week period. The evaluation did not measure wound healing but captured user feedback on dressing application and removal and fluid handling capability.
For the parameters of comfort, exudate management and ease of application and removal, all of the evaluating clinicians allocated a high score of either four or five out of five. When compared to the market leading formulary products, 18 out of 22 indicated the performance of Kliniderm® foam silicone dressing was equivalent. When dressing costs were considered, a potential saving of £27,521 was calculated based on the direct replacement of the two market-leading silicone foam products with Kliniderm® foam silicone, although the author acknowledges that dressing wear time had not been evaluated, and this would need to be considered before making any formulary changes.
In vitro; testing (Carney et al, 2015) examined the free swell capacity (absorption), absorption performance under compression and the amount of force required to peel off the Kliniderm® foam silicone dressing compared to two other market leading products. In all three parameters Kliniderm® foam silicone was comparable with the market leading products.
Exudate management and dressing change
For more information on exudate management using Kliniderm® foam silicone, including tips on when to change the dressing, a mini guide offers further advice: www.hrhealthcare.co.uk/wp-content/uploads/2020/08/3333B-AM121-1-Kliniderm-Exudate-Management_v6.pdf.Management of skin tears
For more information on using Kliniderm® foam silicone in the management of skin tears, including how to draw an arrow on the dressing to indicate the direction of removal from the anchored edge of the skin flap (Figure 3), a mini guide offers further advice: www.hrhealthcare.co.uk/wp-content/uploads/2020/07/3333C-AM122-1-Kliniderm-FS-Skin-Tears_v5.pdf and a skin tear pathway is available www.hrhealthcare.co.uk/wp-content/uploads/2020/07/Kliniderm-Skin-Tear-Pathway-AM093-2-1.pdf.
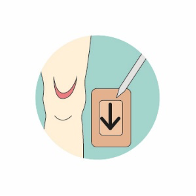
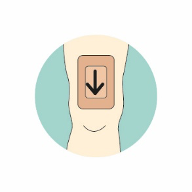
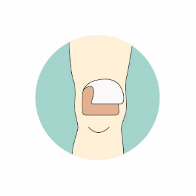
Figure 3. The use of Kliniderm® foam silicone in the management of skin tears.
www.hrhealthcare.co.uk/wp-content/uploads/2020/07/3333A-AM120-1-Kliniderm-FS-PPE_v7.pdf
Carney J, Thomas H, Westgate S, Silabon M (2015) A comparison of a gentle, absorbent silicone foam wound dressing with two market leading products in terms of fluid absorption and peel forces. Perfectus Biomed Ltd, Cheshire. Available online: https://www.hrhealthcare.co.uk/wp-content/uploads/2020/07/A-comparison-of-a-gentle-absorbent-silicone-foam-wound-dressing-with-two-market-leading-products-in-terms-of-fluid-absorption-and-peel-force.pdf
Data on file (2020) KLIN08
Drewery K (2015) Is Kliniderm foam silicone a suitable, cost-saving alternative to other silicone foam dressings? Wounds UK 11(2): 98–103
Drug Tariff (2020) Prices correct from September 2020, based on closest comparable size.
Fumarola S, Allaway R, Callaghan R, et al (2020) Overlooked and underestimated: medical adhesive-related skin injuries. Best practice consensus document on prevention. J Wound Care. 29(Suppl 3c): S1–S24
Jackson L (2019) Skin care following radiotherapy to the breast. Linda Jackson Macmillan Centre. Available online: https://www.ljmc.org/helpful_hints/hhc248_skincare_rt_breast.pdf
Rafter L, Reynolds T, Rafter M (2016) An audit of patient outcomes in the management of skin tears using silicone dressings. Wounds UK 12(2): 70–78
Upton D (2014) Psychological aspects of wound care: implications for clinical practice. J Community Nurs 28(2): 52–7
Upton D, Solowiej K (2010) Pain and stress as contributors to delayed healing. Wound Practice and Research 18(3): 114–22
WUWHS (2007) Principles of best practice: Minimising pain at dressing-related procedures. A consensus document. WoundPedia Inc. Toronto, Ontario, Canada
PPE-related skin damage
For further advice on the use of Kliniderm® foam silicone to provide cushioning against PPE damage a mini guide offers further guidancewww.hrhealthcare.co.uk/wp-content/uploads/2020/07/3333A-AM120-1-Kliniderm-FS-PPE_v7.pdf
Radiation skin damage
Skin care following radiotherapy to the breast is important for patients to be aware of and Jackson (2019) has produced a guide to help them. It is advocated that if there is a moist wound, an absorbent dressing can be used such as a foam dressing. The guide recommends a silicone contact layer and advises the use of Kliniderm® foam silicone as an option.
For more information
Website: www.hrhealthcare.co.uk/portfolio/kliniderm-foam-silicone/
Product enquiries: marketing@hrhealthcare.co.uk
Contact us: +44 (0)1482 631606
Product enquiries: marketing@hrhealthcare.co.uk
Contact us: +44 (0)1482 631606
References
Brown A (2015) The assessment and treatment of wound pain. Nursing Times online. 111(47): 15–17Carney J, Thomas H, Westgate S, Silabon M (2015) A comparison of a gentle, absorbent silicone foam wound dressing with two market leading products in terms of fluid absorption and peel forces. Perfectus Biomed Ltd, Cheshire. Available online: https://www.hrhealthcare.co.uk/wp-content/uploads/2020/07/A-comparison-of-a-gentle-absorbent-silicone-foam-wound-dressing-with-two-market-leading-products-in-terms-of-fluid-absorption-and-peel-force.pdf
Data on file (2020) KLIN08
Drewery K (2015) Is Kliniderm foam silicone a suitable, cost-saving alternative to other silicone foam dressings? Wounds UK 11(2): 98–103
Drug Tariff (2020) Prices correct from September 2020, based on closest comparable size.
Fumarola S, Allaway R, Callaghan R, et al (2020) Overlooked and underestimated: medical adhesive-related skin injuries. Best practice consensus document on prevention. J Wound Care. 29(Suppl 3c): S1–S24
Jackson L (2019) Skin care following radiotherapy to the breast. Linda Jackson Macmillan Centre. Available online: https://www.ljmc.org/helpful_hints/hhc248_skincare_rt_breast.pdf
Rafter L, Reynolds T, Rafter M (2016) An audit of patient outcomes in the management of skin tears using silicone dressings. Wounds UK 12(2): 70–78
Upton D (2014) Psychological aspects of wound care: implications for clinical practice. J Community Nurs 28(2): 52–7
Upton D, Solowiej K (2010) Pain and stress as contributors to delayed healing. Wound Practice and Research 18(3): 114–22
WUWHS (2007) Principles of best practice: Minimising pain at dressing-related procedures. A consensus document. WoundPedia Inc. Toronto, Ontario, Canada



